Anaemia has been acknowledged as a frequent systemic complication and/or extraintestinal manifestation in inflammatory bowel disease (IBD)1 with iron deficiency (ID) being the primary cause of disease2 with an estimated prevalence of iron deficiency anaemia (IDA) among patients with IBD at approximately 45 %.3
Considering the potential effect on hospitalization rates and consequences for patients quality of life, anaemia has been described as a significant and costly complication of IBD.4 However, ID and IDA are under-treated.5
The pathogenesis of ID in IBD is complex, comprising intestinal bleeding, malabsorption, and inadequate oral intake.3 As symptoms of IDA mimic the symptoms of the disease such as fatigue,6 regular screening and diagnosis in these patients are crucial to uncover ID and IDA and therapy of anemia may even improve the quality of life better than the therapy of disease activity.5
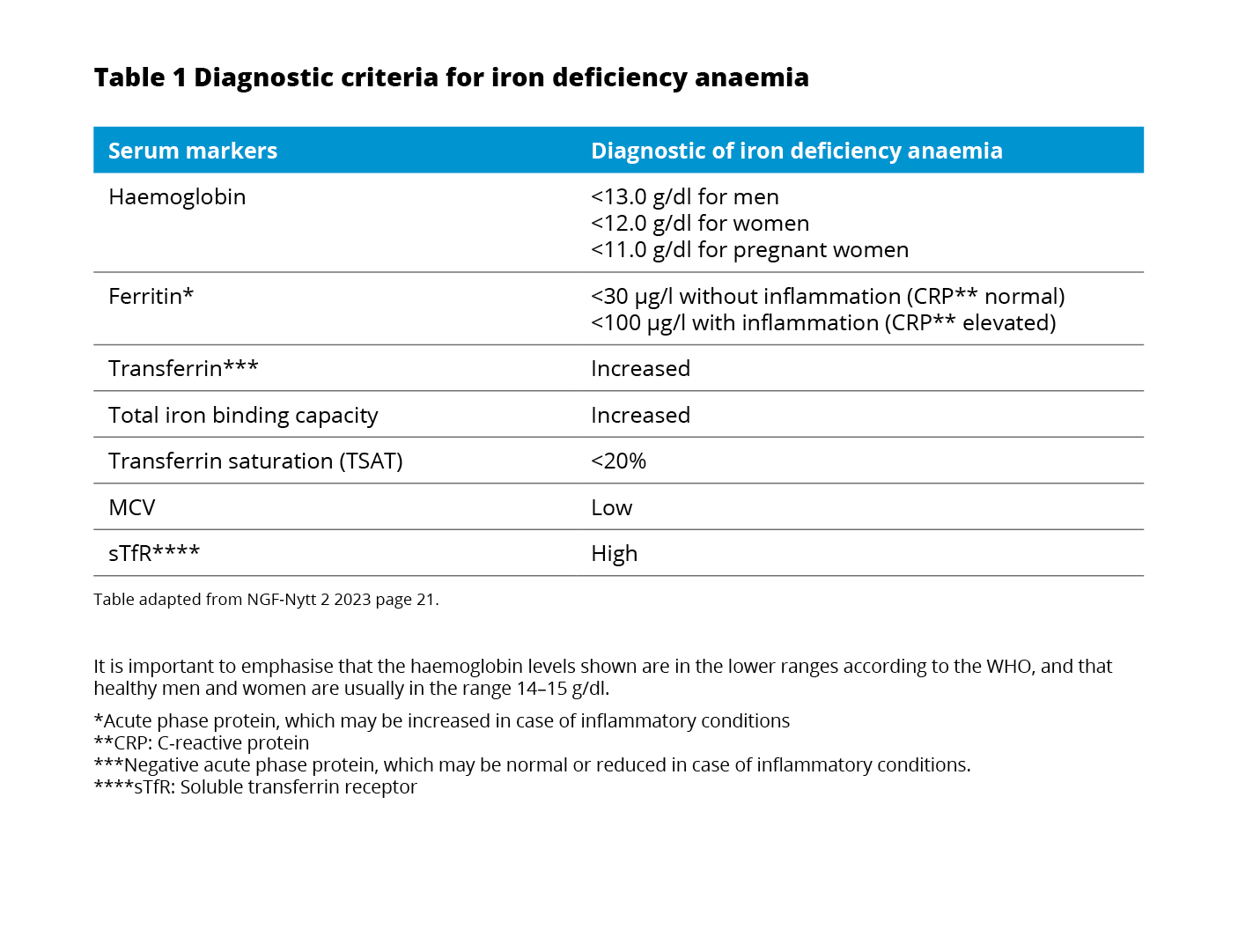
The currently used WHO definition of anaemia7 also applies to patients with IBD.8 All patients with IBD should be assessed for the presence of anaemia through laboratory testing of blood count, serum ferritin, and C-reactive protein every 6-12 months.8
If the hemoglobin (Hb) is below normal, anaemia workup should be initiated including red blood cell distribution width (RDW) and mean corpuscular volume (MCV), reticulocyte count, differential blood cell count, serum ferritin, transferrin saturation (TSAT), and CRP concentration.8
Even when IBD patients are screened, ID and IDA might be overlooked as commonly used laboratory tests may be compromised due to several pathophysiological mechanisms related to the inflammatory nature of IBD9
Consequently, when evaluating ID and IDA in IBD the following mechanisms should be taken into consideration:
Hemoglobin: Iron deficiency should not be excluded despite normal Hb as Hb levels do not decline until a significant amount of iron is lost.10
Ferritin: Besides binding and storing iron in the liver, spleen, and reticuloendothelial system ferritin is also an acute-phase protein and can be increased in the presence of inflammation.11 Following, the interpretation of serum ferritin in patients with inflammation is tricky.5
Hepcidin and ferroportin: The hepatic peptide hormone hepcidin functions as an important regulator of iron homeostasis by controlling ferroportin – the sole iron exporter. However, hepcidin also increases as a response to inflammation.13 The increased levels of hepcidin caused by inflammation will promote the degradation of ferroportin and subsequently impair the exportation of cellular iron into plasma.13
Transferrin saturation: The main iron carrier protein is transferrin.6 In the presence of inflammation, a normal ferritin level does not exclude ID.14 In this case, testing TSAT provides an indication of the extent of iron utilization.10
Soluble transferrin saturation receptor: The TSAT level only indicates the extent of iron utilization.4 A more extensive workup should include soluble transferrin receptor (sTfR) testing.8 sTfR is a truncated form of the cellular transferrin receptor and circulates bound to transferrin.10 It is released in proportion to the expansion of erythropoiesis in the bone marrow and is not regulated by inflammation.5 As a reflector of erythropoiesis, sTfR correlates with the amount of iron available.
Iron supplementation is recommended by The European Chron’s and Colitis Organisation (ECCO) in all IBD patients with IDA with the goal of normalizing hemoglobin levels and iron stores.8
While oral iron substitution is safe, affordable, and easy to administer it might not be the perfect solution for patients with IBD:3
Hence, IV iron therapy is considered first-line treatment for patients with active disease, severe anemia, oral iron intolerance, and erythropoietin requirements.3,8
Komplet produktresumé kan rekvireres vederlagsfrit ved henvendelse til Pharmacosmos A/S.
Lægemiddelform: Jern som ferriderisomaltose - mørkebrun opløsning til injektion/infusion. 1 ml = 100 mg jern tilgængelig som hætteglas eller ampul. Indikationer: Monofer anvendes til behandling af jernmangel når orale jernpræparater ikke kan anvendes eller ved manglende effekt. Samt ved klinisk behov for hurtig tilførsel af jern. Diagnosen skal baseres på laboratorieundersøgelser. Dosering: Max dosis per infusion er 20 mg jern/kg. Max dosis per bolusinjektion er 500 mg jern. Det optimale eller tilsigtede hæmoglobin niveau samt jerndepoter kan variere for forskellige patientgrupper. Der henvises til de officielle retningslinjer. Den kumulative jerndosis beregnes ved hjælp af enten Ganzoni formlen eller forenklet doseringstabel. Det anbefales at anvende Ganzoni formlen til patienter der sandsynligvis vil kræve en individuelt tilpasset dosis, eksempelvis patienter med anoreksi, kakeksi, svær overvægt, graviditet eller anæmi forårsaget af blødning. Administration: Monofer bør kun administreres, når et personale, der har erfaring med at vurdere og håndtere anafylaktiske reaktioner, er umiddelbart tilgængelige i et miljø, hvor der er adgang til komplet genoplivningsudstyr, herunder lægemidler til genoplivning (adrenalin, antihistaminer og/eller kortikosteroider). Patienten skal observeres i mindst 30 minutter efter hver Monofer injektion. Monofer kan indgives uopløst eller opløst i 0,9% natriumkloridopløsning. Hver IV jernadministration er forbundet med risikoen for en overfølsomhedsreaktion. For at minimere risikoen bør antallet af enkelte IV jernadministrationer holdes på et minimum. Doser op til 1000 mg jern indgives over en periode på mindst 15 min – og doser over 1000 mg indgives over en periode på mindst 30 min. Monofer bør ikke anvendes til personer under 18 år. Kontraindikationer: Kendt alvorlig overfølsomhed over for andre parenterale jernpræparater. Anæmi, der ikke skyldes jernmangel. For højt jernniveau eller dårlig udnyttelse af jern. Overfølsomhed overfor det aktive stof, over for Monofer eller øvrige indholdsstoffer. Dekompenseret leversygdom. Særlige advarsler og forsigtighedsregler: Parenteralt administrerede jernpræparater kan forårsage overfølsomhedsreaktioner, herunder alvorlige og potentielt dødelige anafylaktiske reaktioner. Der er også rapporteret overfølsomhedsreaktioner efter tidligere uproblematiske doser af parenterale jernkomplekser. Der har været rapporter om overfølsomhedsreaktioner, som progredierede til Kounis syndrom. Risikoen er øget for patienter med kendte allergier, herunder lægemiddelallergier, hos patienter, der lider af svær astma, eksem eller anden atopisk allergi, og for patienter med andre immunologiske eller inflammatoriske lidelser. Hvis der opstår overfølsomhedsreaktioner eller tegn på intolerans, skal behandlinges stoppes øjeblikkeligt. Hos patienter med kompenseret leverdysfunktion må parenteral jern kun indgives efter en grundig risk/benefit vurdering. Indgivelse af jern skal undgås hos patienter med dysfunktionel lever, hvor jernoverload er en fremskyndende faktor. Nøje monitorering af jernstatus anbefales for at undgå jernoverload. Bør bruges med forsigtighed til patienter med akut eller kronisk infektion. Monofer bør ikke anvendes til patienter, der har bakteriæmi. Hypotension kan opstå, hvis IV injektionen gives for hurtigt. Der skal udvises forsigtighed for at undgå paravenøs lækage ved indgivelse af Monofer. Interaktioner: Monofer bør ikke gives samtidigt med orale jernpræparater, da absorptionen af oralt jern kan reduceres. Kan medføre falske, forhøjede værdier af serum bilirubin og falske, lave værdier af serum kalcium. Graviditet: Der er kun begrænsede data fra brugen af Monofer hos gravide kvinder fra en undersøgelse med 100 eksponerede gravide kvinder. Det er derfor nødvendigt omhyggeligt at vurdere fordele og ulemper inden brug under graviditet. I sjældne tilfælde er der hos gravide kvinder med overfølsomhedsreaktioner observeret føtal bradykardi. Amning: kan anvendes. Bivirkninger: Almindelige (≥1/100 til <1/10): Kvalme, reaktioner på injektionsstedet. Ikke almindelige (≥1/1000 til <1/100): Overfølsomhed inkl. alvorlige reaktioner, hovedpine, paræstesi, dysgeusi, sløret syn, bevidsthedstab, svimmelhed, træthed, takykardi, hypotension, hypertension, brystsmerter, åndenød, bronkospasmer, abdominale smerter, opkastning, dyspepsi, forstoppelse, diarré, kløe, nældefeber, udslæt, rødme, øget svedafsondring, dermatitis, hypofosfatæmi, rygsmerter, myalgi, artralgi, muskelkramper, pyreksi, kulderystelser/rysten, infektion, lokal flebitisk reaktion, forhøjede leverenzymer. Sjældne (≥1/10000 til <1/1000): anafylaktiske reaktioner, dysfoni, tremor, ændret mental status, arytmi, angioødem, influenza lignende symptomer. Dato for SPC: 2022-11-11. Udleveringsgruppe: B. ATC: B03AC. Pakningsstørrelser: 5x1 ml, 5x5 ml, 2x10 ml. Dagsaktuel pris kan findes på www.medicinpriser.dk. Det fulde produktresumé kan læses på www.produktresume.dk Indehaver af markedsføringstilladelsen: Pharmacosmos A/ S, Rørvangsvej 30, DK-4300 Holbæk.
Kontakt os - E-mail: info@pharmacosmos.com
Tlf. Nr.: +45 5948 5959
Hjemmeside: www.pharmacosmos.com/nordic
07-SCAN-11-09-2023.v1
PHOSPHARE-IBD study presentation
Gastro advertorial Denmark
Cardio advertorial Denmark
Gastroenterology article 2